Introduction: Emicizumab (Hemlibra: Roche Switzerland) is a, humanized, bi-specific monoclonal modified immunoglobulin G4 (IgG4) which binds human FX, FIX and activated FIX (FIXa) to imitate activated FVIII exercise.
Intention: Consider the consequences of emicizumab on the APTT, surrogate FVIII exercise and FVIII inhibitor outcomes.
Strategies: Two samples had been offered, one obtained from an emicizumab handled extreme haemophilia A affected person with FVIII inhibitors and one constructed by in vitro addition of emicizumab utilizing plasma from a extreme haemophilia A affected person with out FVIII inhibitors. An APTT display, surrogate FVIII and FVIII inhibitor exams had been carried out on each samples by taking part centres.
Outcomes: APTT outcomes had been under the decrease restrict of regular vary. Chromogenic FVIII assay outcomes with the Hyphen/Biophen human component-based assay gave larger than anticipated coefficient of variation (CV) outcomes, 38%-40%. The modified one-stage FVIII assay with emicizumab calibrators confirmed related outcomes whatever the APTT reagent. Inhibitor assay median outcomes for pattern S18:23 = 1.43 BU (vary 0.9-3.Zero BU/ml, CV 38%). S18:24 was categorised as under the decrease restrict of detection.
Conclusion: APTT screens confirmed a constant shortening. Unmodified one-stage Issue VIII assay outcomes had been remarkably excessive. APTT-based assays are unsuitable for measurement of coagulation elements or inhibitors in sufferers handled with emicizumab. Bovine origin chromogenic assays are insensitive to emicizumab and ought to be used to observe FVIII ranges/FVIII inhibitors in emicizumab handled sufferers. Product-specific calibrators ought to be carried out to cut back outcome variability.
Identification of Immunohistochemical Reagents for In Situ Protein Expression Evaluation of Coronavirus-associated Adjustments in Human Tissues
We studied the suitability of commercially accessible monoclonal antibodies (mAbs) for the immunohistochemical (IHC) detection of extreme acute respiratory syndrome coronavirus 2 (SARS-CoV2) in customary archival specimens. Antibodies had been screened on HEK293 cells transfected with viral nucleoprotein, S1 subunit and S2 subunit of spike protein and on untransfected cells, in addition to a panel of regular tissue.
Lung tissue with presence of SARS-CoV2 confirmed by in situ hybridization (ISH) was additionally used. A complete of seven mAbs had been examined: (1) mAb 001 (Sino Organic, 40143-R001), (2) mAb 007 (Sino Organic, 40150-R007), (3) mAb 019 (Sino Organic, 40143-R019), (4) mAb 1A9 (GeneTex, GTX632604), (5) mAb ABM19C9 (Abeomics, 10-10007), (6) FIPV3-70 (Santa Cruz, SC-65653), and (7) mAb 6F10 (BioVision, A2060). Solely 2 mAbs, clone 001 to the nucleoprotein and clone 1A9 to the S2 subunit spike protein displayed particular immunoreactivity.
Each clones confirmed robust staining within the acute section of COVID-19 pneumonia, principally in areas of acute diffuse alveolar injury, however weren’t utterly congruent. Viral protein was additionally present in kidney tubules, endothelia of a number of organs and a nasal swab of a affected person with persistent SARS-CoV2 an infection.
The opposite examined reagents had been both poorly reactive or demonstrated nonspecific staining in tissues and lesions not contaminated by SARS-CoV2. Our examine demonstrates that inflexible specificity testing is necessary for the analysis of mAbs to SARS-CoV2 and that clones 001 to nucleoprotein and 1A9 to S2 subunit spike protein are helpful for the in situ detection of SARS-CoV2.
An Improved Protocol for the Synthesis and Purification of Yariv Reagents
Yariv reagents are glycoconjugate tris-azo dyes broadly utilized in plant biology. These reagents are synthesized by diazo coupling between phloroglucinol and a para-diazophenyl glycoside. Regardless of their artificial accessibility, well-defined protocols for acquiring pure Yariv reagents, and their full compound characterization information, haven’t been reported.
We report right here optimized protocols used to synthesize, purify, and characterize a panel of six Yariv reagents and recommend approaches that might be worthwhile for the purification and characterization of different glycoconjugates as effectively.
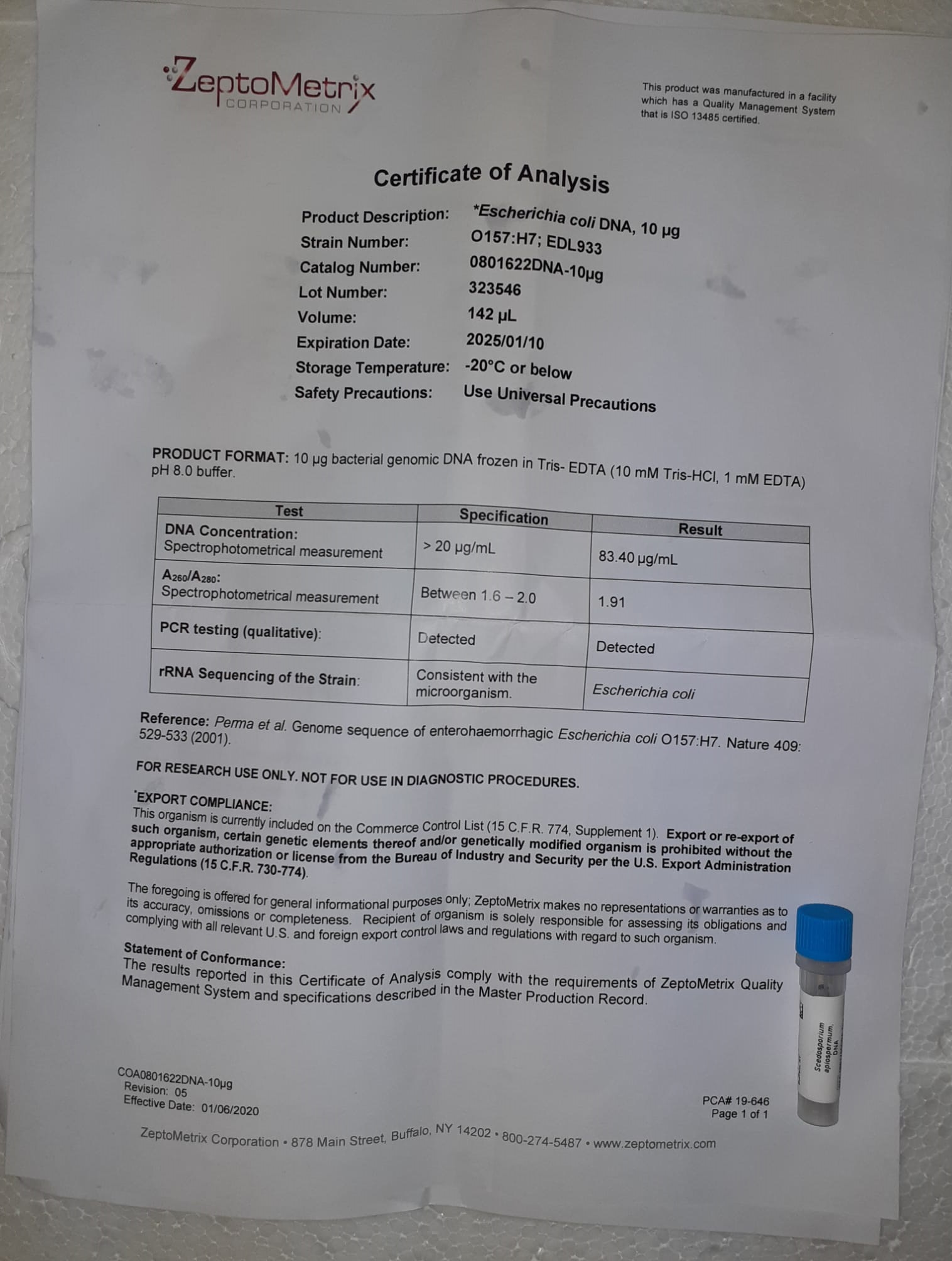
Analysis of STA-NeoPTimal, an extraction thromboplastin reagent with ISI near 1
Introduction: The prothrombin time (PT) is essentially the most requested take a look at to analyze sufferers with congenital or acquired coagulopathies or to observe oral anticoagulant remedy. Nevertheless, thromboplastins can present markedly completely different responsiveness to the defects induced by vitamin Okay antagonist (VKA) remedy and are thus characterised by their ISI (Worldwide Sensitivity Index). INR outcomes are optimum for sufferers beneath VKA however for sufferers screened for different causes expressing PT outcomes as ratio could be extra applicable. As it is vitally tough to outline the PT outcomes reporting unit from the PT testing request, it will be supreme to make use of a thromboplastin with ISI = 1. The examine goals to check our reference PT reagent with two candidate thromboplastins with ISI near 1.
Strategies: We in contrast Three completely different thromplastins: two rabbit mind extracted primarily based reagents (STA-Neoplastine CI Plus, with ISI = 1.26, routinely utilized in our laboratory and STA-NeoPTimal with ISI = 1.01) and a recombinant thromboplastin (STA-Neoplastine R with ISI = 0.97). The comparability was executed on 175 samples: 75 from people with out coagulation defects and 100 from sufferers beneath VKA.
Outcomes: STA-NeoPTimal and STA-Neoplastine R effectively correlate to our reference, STA-Neoplastine CI Plus: regression equations are y = 1.186x-0.1351, r2 = .9454 and y = 1.1432x-0.1554, r2 = .9951, respectively. The bottom bias on INR outcomes was obtained with STA-NeoPTimal reagent (interval: -0.7/+0.4).
Conclusion: We conclude that STA-NeoPTimal can be utilized within the laboratory because it provides outcomes akin to these obtained with STA-Neoplastine CI Plus. Moreover, due to its ISI = 1, it ensures reporting a PT ratio equal to INR which avoids errors.
heraeus-targets
Improvement of α,α-Disubstituted Crotylboronate Reagents and Stereoselective Crotylation through Brønsted or Lewis Acid Catalysis
The event of α,α-disubstituted crotylboronate reagents is reported. Chiral Brønsted acid-catalyzed uneven aldehyde addition with the developed E-crotylboron reagent gave (E)-anti-1,2-oxaborinan-3-enes with wonderful enantioselectivities and E-selectivities. With BF3·OEt2 catalysis, the stereoselectivity is reversed, and (Z)-δ-boryl-anti-homoallylic alcohols are obtained with wonderful Z-selectivities from the identical E-crotylboron reagent.
The Z-crotylboron reagent additionally participates in BF3·OEt2-catalyzed crotylation to furnish (Z)-δ-boryl-syn-homoallylic alcohols with good Z-selectivities. DFT computations set up the origins of noticed enantio- and stereoselectivities of chiral Brønsted acid-catalyzed uneven allylation. Stereochemical fashions for BF3·OEt2-catalyzed reactions are proposed to rationalize the Z-selective allyl additions. These reactions generate extremely worthwhile homoallylic alcohol merchandise with a stereodefined trisubstituted alkene unit. The artificial utility is additional demonstrated by the whole syntheses of salinipyrones A and B.